Abstract
Background
Eculizumab, the first C5 inhibitor approved for paroxysmal nocturnal hemoglobinuria (PNH), transformed PNH treatment by improving survival to that of an age- and sex- matched general population. Previous analyses demonstrating the survival benefit of eculizumab in patients with PNH leveraged historical data and were limited by small patient numbers and short follow-up durations; few evaluated survival of patients receiving eculizumab compared with untreated patients. The objective of the current analysis was to describe the baseline characteristics and overall survival of a large international cohort of eculizumab-treated patients compared with a contemporaneous untreated cohort using data from the prospective, observational International PNH Registry (NCT01374360).
Methods
Data from patients enrolled in the Registry after March 16, 2007 with complete information for birth date, sex, enrollment date, and treatment status were included (database cut-off, April 12, 2021). Ever-treated patients were those who received eculizumab for a minimum treatment period of 35 days while enrolled in the Registry; never-treated patients did not receive eculizumab at any time before or during Registry participation. Univariate and multivariate analyses were performed using a Cox proportional hazards that incorporated the following parameters at baseline as covariates: treatment status, presence of high disease activity (HDA), age, sex, history of bone marrow failure (BMF), history of thrombotic events (TE), transfusion dependence, and estimated glomerular filtration rate ≤60 mL/min/1.73 m 2. HDA was defined as lactate dehydrogenase (LDH) ratio ≥1.5 × upper limit of normal (ULN) and ≥1 of the following: history of major adverse vascular events (including TE); anemia (hemoglobin <10 g/dL), or physician-documented abdominal pain, dyspnea, dysphagia, fatigue, hemoglobinuria, or erectile dysfunction at any time before and including baseline. Baseline was defined as the date of eculizumab treatment initiation (ever-treated patients) or date of Registry enrollment (never-treated patients). Survival time was analyzed using a left-truncation approach that mapped time in patients' survival based on disease start date, defined as the earliest date of first-reported PNH diagnosis, PNH symptom, or first consistent flow cytometry result.
Results
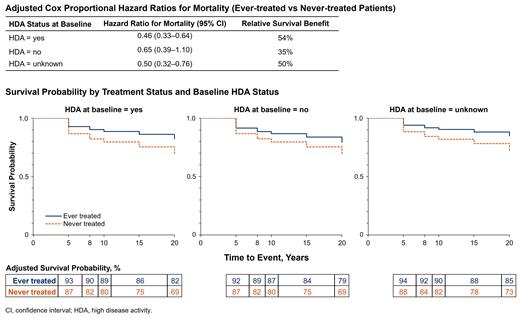
Baseline characteristics of the 4627 patients included in the analysis (mean [SD] age at disease start, 40.2 [18.71] years; 53% female; 75% white) were comparable between the ever-treated and never-treated groups (n=1892 and n=2735, respectively). Compared with never-treated patients, more ever-treated patients had LDH ≥1.5 × ULN (90% vs 35%), and fewer had <10% PNH granulocytes (3% vs 57%) or history of BMF (45% vs 76%). The univariate Cox proportional hazard ratio (HR) for mortality in ever-treated vs never-treated patients was 0.48 (95% CI, 0.39-0.60; P<0.0001), indicating a 52% increase in survival in the treated group (Table). Among ever-treated patients, those with HDA at baseline experienced the largest reduction in mortality risk (HR [95% CI], 0.46 [0.33-0.64]; n=174); however, decreased mortality was also evident in ever-treated patients without HDA (HR, 0.65 [0.39-1.10]; n=212) or with unknown HDA status (HR, 0.50 [0.32-0.76; n=120) at baseline. Overall survival probability by treatment status was consistently greater in ever-treated vs never-treated patients through 20 years of follow-up; survival probability at 20 years was 82% (ever-treated) vs 69% (never-treated). Although long-term survival probability was greatest throughout follow-up in ever-treated patients with HDA at baseline, increased survival among ever-treated patients was evident in all 3 HDA status groups (Figure).
Conclusion
In this analysis of Registry data, treatment with the C5 inhibitor eculizumab improved patient survival compared with a never-treated cohort at a comparable time point in their disease course. Covariates were assessed at baseline only and competing risks and time on treatment were not controlled for, which are potential limitations. Survival benefits conferred by eculizumab treatment were observed regardless of HDA status at baseline, were more pronounced in treated patients with HDA vs those without HDA, and were maintained through 2 decades of real-world follow-up.
Terriou: Alexion, AstraZeneca Rare Disease: Consultancy, Membership on an entity's Board of Directors or advisory committees. Patriquin: Alexion, AstraZeneca Rare Disease: Consultancy, Honoraria, Speakers Bureau; Biocryst: Honoraria; Apellis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria. Griffin: Alexion, AstraZeneca Rare Disease: Honoraria, Membership on an entity's Board of Directors or advisory committees; BioCryst Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sobi Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Apellis: Other: Educational grant support. Lee: Alexion, AstraZeneca Rare Disease: Honoraria, Membership on an entity's Board of Directors or advisory committees. Gustovic: Alexion, AstraZeneca Rare Disease: Current Employment. Patel: Alexion, AstraZeneca Rare Disease: Current Employment. Szer: Alexion, AstraZeneca Rare Disease: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Apellis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Prevail Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal